BPOM Supervision Officially Applies to PSE/PPMSE:A Guide to BPOM Regulations No. 14 of 2024
Authors
On 5 August 2024, Indonesia’s Food and Drug Authority (Badan Pengawas Obat dan Makanan - “BPOM”) enacted Regulation No. 14 of 2024 on the Supervision of Drugs and Food Distributed Online (“BPOM Reg 14/2024”), replacing Regulation No. 8 of 2020.
This new regulation aims to protect consumers by ensuring the safe online distribution of various products, including drugs, medicinal ingredients, quasi-drugs, health supplements, cosmetics, processed foods, Processed Foods for Special Medical Purposes (Pangan Olahan untuk Keperluan Medis Khusus - “PKMK”), and Food Additives (Bahan Tambahan Pangan - “BTP”).
Obligations and Limitations for Businesses
Under Indonesian law, Businesses (or Companies) refer to any entities involved in commercial activities, including manufacturers, distributors, and service providers. In the context of BPOM Reg 14/2024, this term includes:
• Drugs and Food Businesses: Companies involved in producing, distributing, or selling drugs, food, health supplements, and related products.
• PSE (Electronic System Providers – Penyelenggara Sistem Elektronik): Every person, state administrators, business entities, and communities that provide, manage, and operate electronic systems to electronic system users for their own purposes and/or the purposes of other parties.
• PPMSE (Electronic Commerce Operators - Penyelenggara Perdagangan Melalui Sistem Elektronik): Companies that directly handle the sale of goods or services online. An example would be e-commerce, which manage both the platform and the actual transactions for products sold on its site.
Based on Article 6 through Article 7 of BPOM Reg 14/2024, these Businesses are required to comply with the following obligations and limitations:
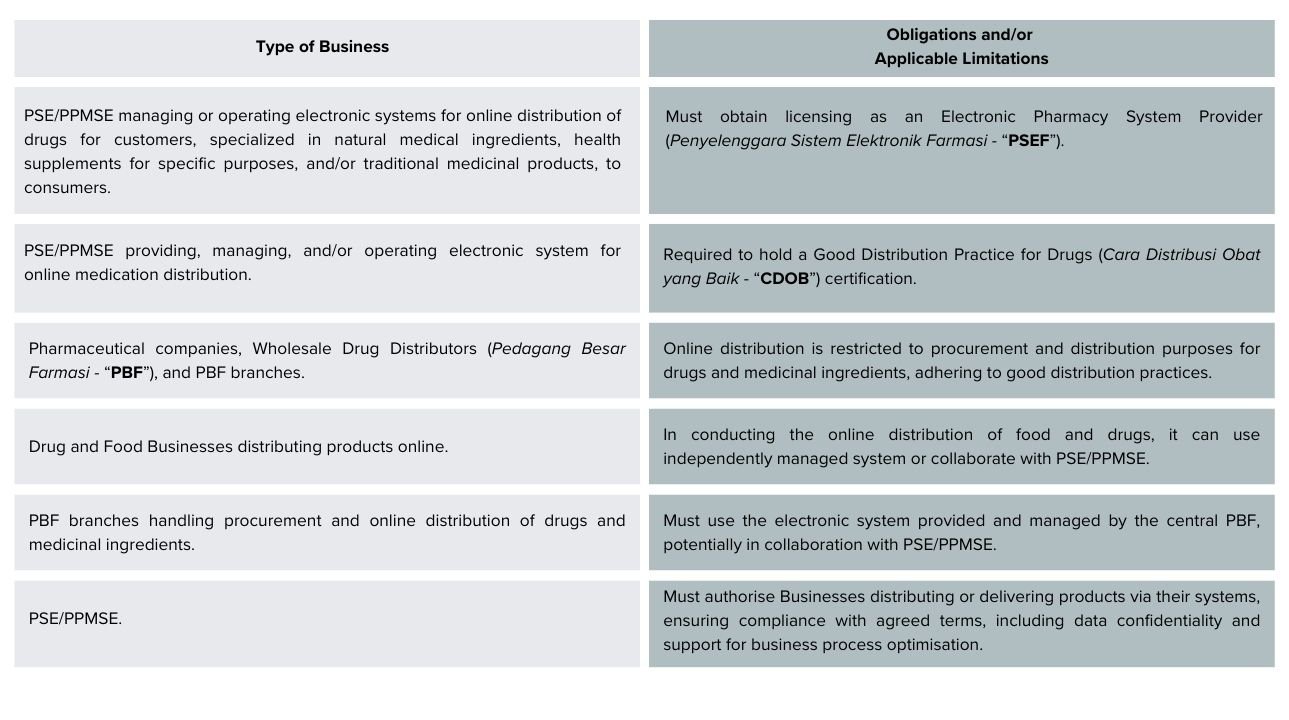
In this context, e-commerce is not PSE/PPMSE in the category mentioned in BPOM Reg 14/2024 and therefore does not require licensing as PSEF or obtaining CDOB certificates. To be licensed as a PSEF, a PSE/PPMSE must be an authorized and licensed pharmacy with a pharmacist.
Furthermore, one of the requirements to obtain CDOB certificate is that Companies have at least a pharmacist in charge. In this context, Companies that require CDOB certificate are drugs distributors.
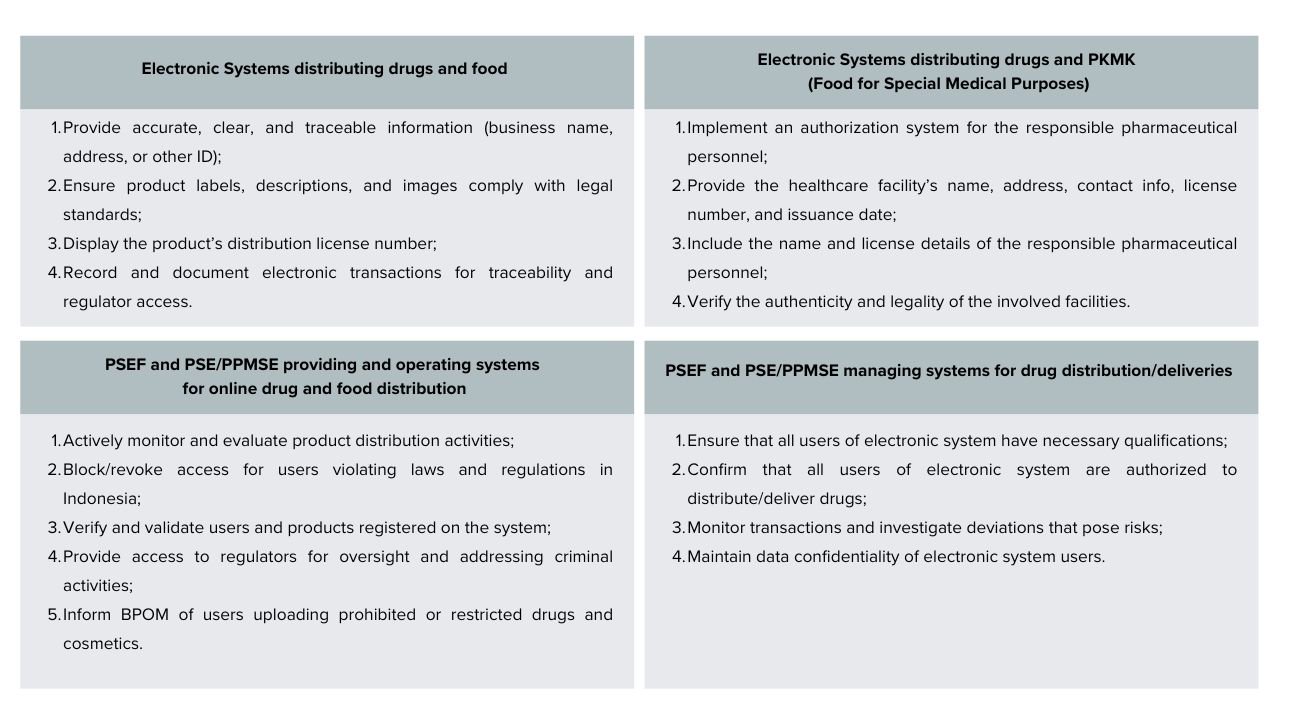
Requirements for the Use of Electronic Systems
In addition to the obligations for Businesses, BPOM Reg 14/2024 sets requirements for the electronic systems used in the distribution of drugs and food:
Reporting Obligations
Entities involved in the online distribution of drugs and medicinal ingredients (including pharmaceutical companies, PBF, and PBF branches), are required to:
(a) Report their activities to the Head of BPOM, specifying the name of the electronic system used before submitting the report.[1]
(b) Provide these reports at the time an account is created in the electronic system.[2]
(c) Notify BPOM monthly of any changes in the previously reported information (applicable to PSEF and PSE/PPMSE).[3]
Administrative Sanction
According to Article 27 of BPOM Reg 14/2024, Businesses and/or the third parties who violate the provisions of this regulation may face administrative sanctions, including:
a. Warning;
b. A stern warning;
c. Temporary suspension on distribution activities; and/or
d. Recall order for drugs and food.
Disclaimer:
This client update is the property of ARMA Law and intended for providing general information and should not be treated as legal advice, nor shall it be relied upon by any party for any circumstance. ARMA Law has no intention to provide a specific legal advice with regard to this client update.
Related Updates
Latest Updates


